Predict the Molecular Geometry and Polarity of the So2 Molecule.
It has a difference in electronegativity values between sulfur and oxygen atoms with oxygens pull the electron. Predict the molecular geometry and polarity of the CS2 molecule.

So2 Molecular Geometry Shape And Bond Angles Sulfur Dioxide Youtube
Let us look at the periodic table.

. SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms. There are two Oxygen atoms bonded to. Using this information we can describe the molecular geometry The arrangement of the bonded atoms in a molecule or a polyatomic ion in space the arrangement of the bonded atoms in a.
A molecule with 2 single bonds and 2 lone pairs of electrons around the central atom is predicted to have what type of molecular geometry. The greater the difference in. Sulfur brings 6 and oxygen brings 3 each.
It is used in Riley reactions as a starting material and is vital in the synthesis of. Chemistry questions and answers. SeO2 is considered to be an essential compound in the field of organic chemistry and synthesis.
A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. The lone pair of electrons is at the top of the SO2 molecule. A three-step approach for drawing the SO3 molecular can be used.
To determine the molecular geometry of Sulfur Dioxide we must observe its Lewis structure. SO2 molecular geometry is tilted at 119 degrees bond angle of O-S-O. Predict the molecular geometry and polarity of the SO2 molecule.
Thus the dipole moment is a tool to predict the polarity of a. SO2 a Bent molecular shape. SO2 Molecular Geometry and Shape.
To correctly determine the molecular shape of a molecule requires that you first draw the Lewis structure. Nitrogen belongs to group 15. Lewis Structure of NO2.
Predict the geometry and polarity of the CS2. Predict the molecular geometry and polarity of the SO2 molecule by applying VSEPR theory. The SO2 bond angle will be 120 degrees since it has a Bent.
Non-polar bonds or non-polar molecules do not have dipole moments and polar bonds or polar molecules have dipole moments. Bent 120 degree bond angle polar Predict the molecular geometry and polarity of the O---C---S. An atom or molecules tendency to attract electrons and thus form bonds.
A link or force between neighboring atoms in a molecule. O linear polar linear nonpolar O tetrahedral nonpolar bent nonpola O bent polar. Predict the molecular shape and give the approximate bond angles in the PCl3 molecule.
Here sulfur in the center because of its lowest electron capability and three oxygen around it. A linear 180º B trigonal planar 120º C tetrahedral 1095º. Lewis dot structures can lead to a reasonable prediction of molecular structure.
The first step is to sketch the molecular geometry of the SO3 molecule to calculate the lone pairs of the electron in the. For example in the first question SO2 has a lone pair of electrons on sulfur whereas carbon in CO2 does not. Is SO2 polar or nonpolar.
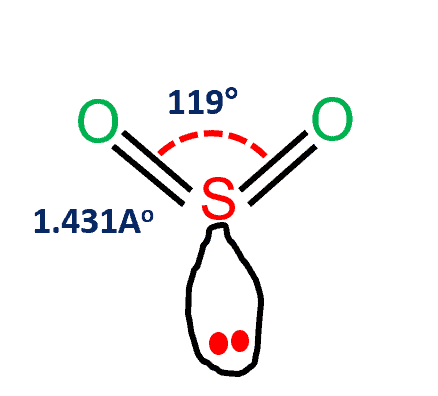
So2 Polar Or Nonpolar What S Insight

Solved 14 Predict The Molecular Geometry And Polarity Of Chegg Com

So2 Molecular Geometry Science Education And Tutorials
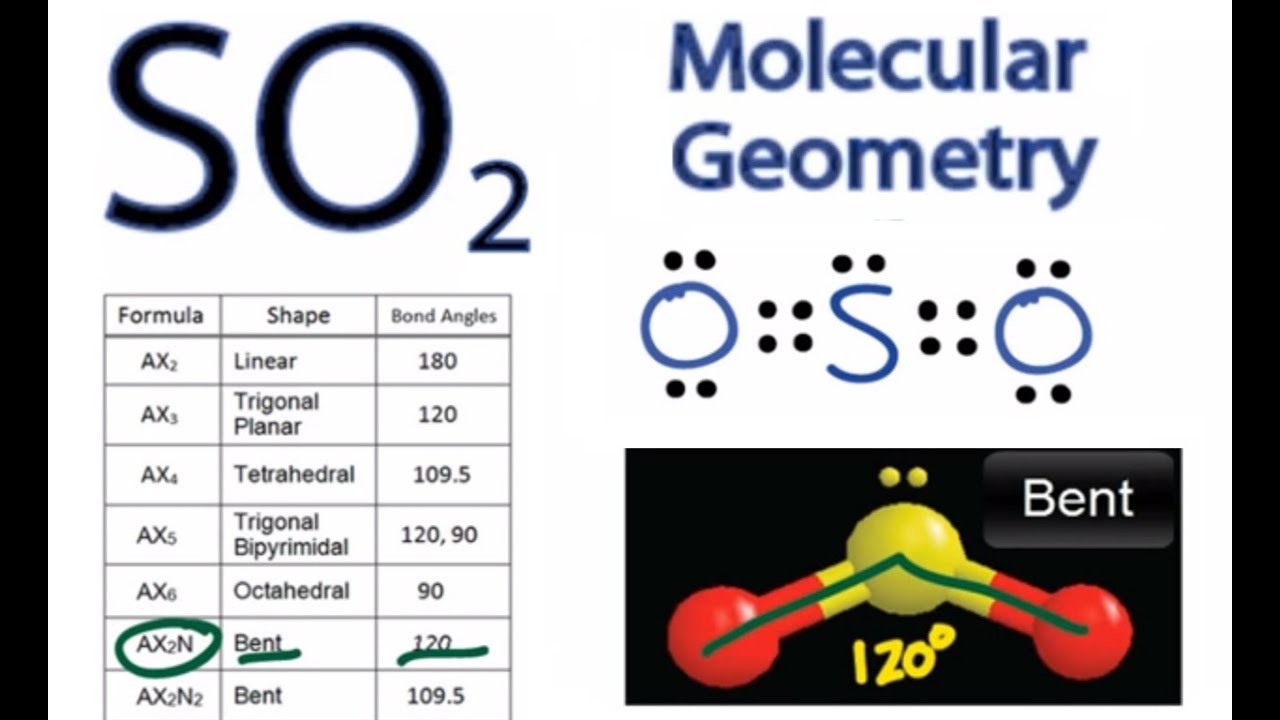
So2 Molecular Geometry Shape And Bond Angles Sulfur Dioxide Youtube
No comments for "Predict the Molecular Geometry and Polarity of the So2 Molecule."
Post a Comment